What Charge Do Neutrons Have
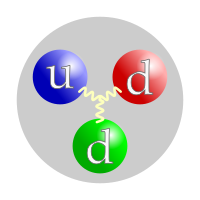
A movie of a neutron. The 'u' stands for an upwardly quark, and the 'd' stands for a down quark.
Neutrons, with protons and electrons, make upwardly an atom. Neutrons and protons are institute in the nucleus of an cantlet.[1] [2] [3] Unlike protons, which take a positive charge, or electrons, which take a negative charge, neutrons have zero charge[1] [iv] which ways they are neutral particles. Neutrons bind with protons with the residual potent force.
Neutrons were predicted by Ernest Rutherford,[v] and discovered by James Chadwick,[6] [7] in 1932.[six] Atoms were fired at a thin pane of beryllium. Particles emerged which had no charge, and he called these 'neutrons'. They were afterward added to the modern image of the atom.
Neutrons have a mass of one.675 × 10-24chiliad,[8] which is a footling heavier than the proton.[8] Neutrons are 1839 times heavier than electrons.[viii]
Like all hadrons, neutrons are made of quarks. A neutron is made of two downward quarks and one upward quark.[two] [three] Ane upward quark has a charge of +two/3, and the ii down quarks each accept a charge of -one/3. The fact that these charges cancel out is why neutrons have a neutral (0) accuse. Quarks are held together by gluons.
Isotopes [modify | change source]
Neutrons tin can exist establish in almost all atoms together with protons and electrons. Hydrogen-1 is the only exception. Atoms with the same number of protons but a different number of neutrons are called isotopes of the same element.
The number of neutrons in an atom does not affect its chemical properties. However it affects its half-life, a measure out of its stability. An unstable isotope has a short half-life, in which half of it decays to lighter elements. By contrast, a stable isotope has a long one-half-life, much longer than that of an unstable isotope. The stability of an isotope is related to radioactivity: an unstable isotope can be highly radioactive.
Atomic reactions [change | change source]
Neutrons are the primal to nuclear chain reactions, nuclear power and nuclear weapons.
[change | modify source]
- Proton
- Electron
References [alter | change source]
- ↑ i.0 one.1 Woolley, Steve (2011). Edexcel IGCSE Physics Revision Guide. Pearson Instruction. p. twenty-21. ISBN9780435046736.
- ↑ 2.0 2.1 Cox, Brian; Cohen, Andrew (2011). Wonders of the Universe. HarperCollins. p. 79, 108. ISBN9780007395828.
- ↑ Ryan, Lawrie (2001). Chemical science for you: revised National Curriculum edition for GCSE (Second ed.). Nelson Thornes. p. 29. ISBN9780748762347.
- ↑ "Ernest Rutherford". chemed.chem.purdue.edu.
- ↑ 6.0 6.one "Discovery of Neutrons". Helmholtz Zentrum Berlin. 2008-08-23. Archived from the original on 2010-11-13. Retrieved 2011-04-14 .
- ↑ "The Nobel Prize in Physics 1935: James Chadwick". Nobelprize.org. Retrieved 2011-04-15 .
- ↑ eight.0 viii.ane 8.2 "Neutron (subatomic particle)". Encyclopædia Britannica Online. Encyclopædia Britannica. Retrieved 2011-04-xiv .
What Charge Do Neutrons Have,
Source: https://simple.wikipedia.org/wiki/Neutron
Posted by: petersonwhichoune.blogspot.com


0 Response to "What Charge Do Neutrons Have"
Post a Comment